China-Latin America: COVID-19 Vaccine Collaboration and the Way Forward
July 2, 2021 | By Zixiang (George) Zhou, Associate, Bridge Consulting; Marina Figueira, Junior Associate, Global Health Strategies Brazil; Marisa Lim, 2021 Spring Intern, Bridge Consulting.
Key Takeaways
- Unprecedented vaccine partnerships between China and Latin America have given millions of people in Latin America access to life-saving COVID-19 vaccines.
- By understanding each other’s capacities, concerns, and needs and enlisting more help from international organizations, stakeholders from China and Latin America can achieve more.
- To resolve vaccine inequity for COVID-19 and beyond, all stakeholders involved should support joint vaccine R&D and help build regional vaccine manufacturing hubs in Latin America.
China and Latin America have built important partnerships during the COVID-19 pandemic through South-South Cooperation, with Latin America being the second biggest buyer and recipient of Chinese COVID-19 vaccines worldwide (Figure 1) and having the second most vaccine manufacturing deals with China. While the Chinese, Latin American and international stakeholders involved have very different capacities, needs and concerns, they have proved through current partnerships that innovative collaboration in vaccine R&D and production is indeed possible, with the potential to do even more.
Amidst a COVID-19 vaccine shortage plaguing many countries around the world, the call for greater international cooperation in vaccine R&D and production to ensure equitable distribution of COVID-19 vaccines has never been more crucial. This is an opportune time for Chinese, Latin American and international stakeholders to work together to improve their mutual understanding, expand and deepen collaboration, and develop solutions to secure vaccine inequity for COVID-19 and beyond.
How have China and Latin America engaged in COVID-19 vaccine partnerships?
Chinese, Latin American and international stakeholders have successfully collaborated in the COVID-19 vaccine R&D, production, and real-world testing of the vaccines.
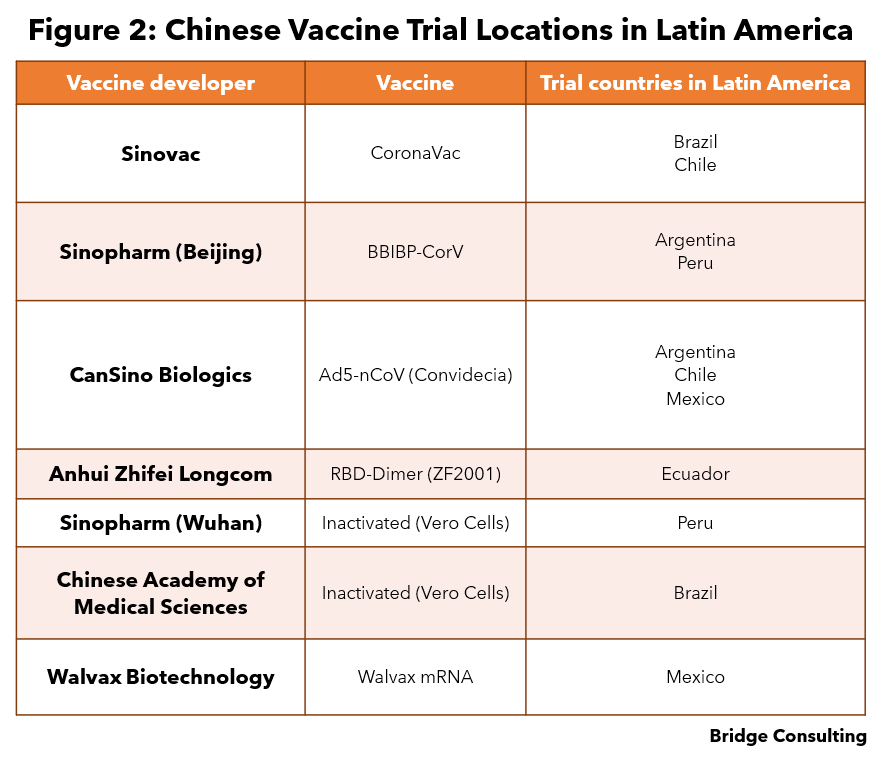
Vaccine R&D. Several Latin American countries have hosted clinical trials for various Chinese vaccine developers (Figure 2). Trial locations also tend to see quicker approvals for use as well as larger purchase quantities and faster deliveries of the vaccines.
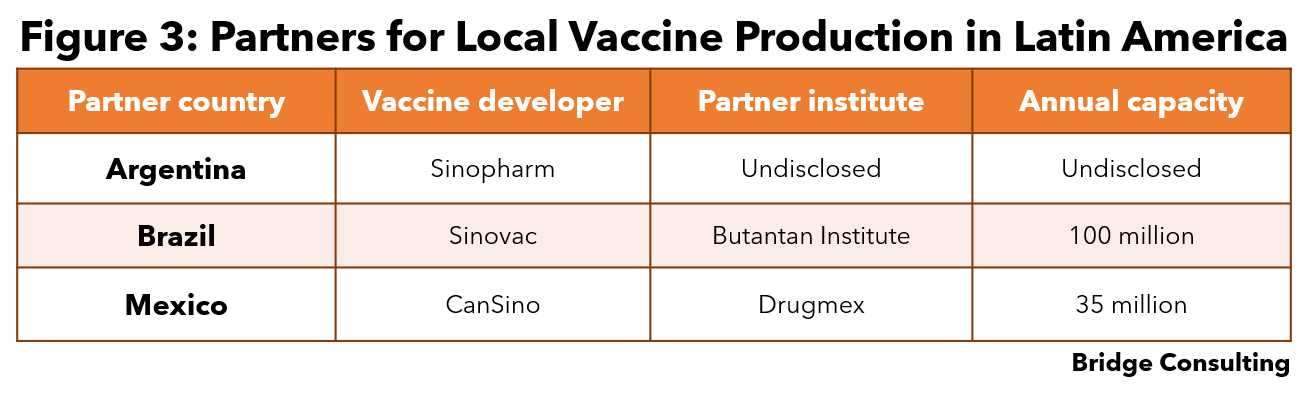
Vaccine production. Argentina, Brazil, and Mexico are currently partnering with Sinopharm, Sinovac, and CanSino respectively to manufacture these vaccines locally (Figure 3). Brazil has signed technology transfer agreements for the AstraZeneca and the CoronaVac vaccines, which are currently packaged in Brazil with raw ingredients from China.
China: Capacities, Needs and Concerns
Who are the Chinese Stakeholders?
Chinese stakeholders have been working on COVID-19 vaccine R&D and production since the beginning of the pandemic.
The Chinese Government. Scientists at the Chinese Academy of Science and other government research institutes have worked with vaccine developers to develop several COVID-19 vaccines. Chinese government agencies at various levels have introduced measures to provide policy and funding support to vaccine R&D and production in China as well as international cooperation in this area. For example, Sinovac used designated low interest loans provided by the People’s Bank of China, China’s central bank, to fund the expansion of its COVID-19 vaccine production capacity.
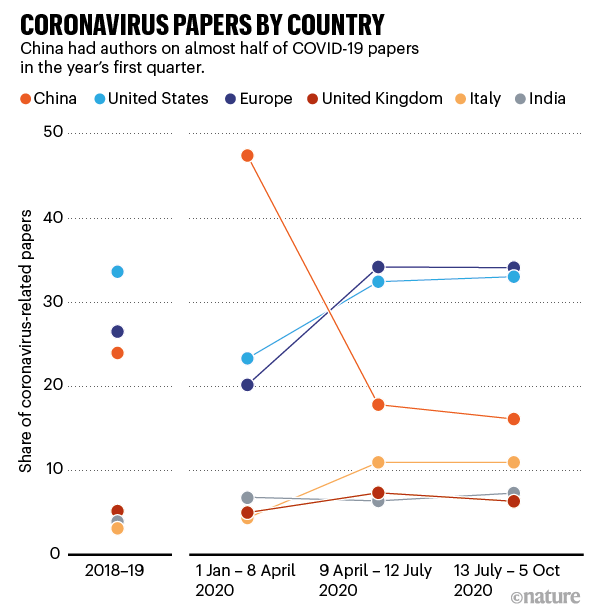
Non-profit organizations and scientific community. According to the Pandemic Philanthropy Report published by Bridge Consulting in June 2020, the total cash contributions by Chinese philanthropy to COVID-19 R&D including vaccine R&D are estimated at CNY 1.8 billion (USD 256 million). Non-profit organizations such as the Jack Ma Foundation and the Tencent Charity Foundation have donated millions to fund COVID-19 vaccine R&D in and outside of China. Chinese scientists have also contributed a great deal of scientific findings on SARS-CoV-2 and COVID-19 vaccines, while benefiting from findings made by scientists from other countries (Figure 5).
What is China’s Capacity Like?
Vaccine R&D and Manufacturing Capacities. As a country with a rapidly expanding vaccine industry, China has over time developed the capacity to produce all vaccines in its national immunization program. The two WHO-approved Chinese COVID-19 vaccines developed by Sinopharm and Sinovac again demonstrated China’s vaccine R&D capacity. Chinese vaccine developers also have experience developing vaccines in response to outbreaks. Sinovac developed a vaccine against Severe Acute Respiratory Syndrome (SARS) for human trial in 2003. CanSino working with Chinese Academy of Military Medical Sciences’ Bioengineering Institute developed the first Ebola vaccine approved by China and tested it during the Ebola outbreak in West Africa.
Funding Capacity. Chinese President Xi Jinping has pledged billions of dollars in international aid to support COVID-19 response in other developing countries and made it clear that China supports its vaccine companies in transferring technologies to other developing countries and carrying out joint production with them.
What are their Concerns and Needs?
Lack of Local Knowledge. Chinese vaccine developers have very limited previous experience exporting its vaccines to Latin America. Chinese vaccine developers need support from the local governments as well as R&D and manufacturing partners. They also need local partners to help them better understand and communicate with the stakeholders and public in Latin America.
Funding and Policy Restraints. Chinese vaccine developers need more incentives and support to continue to expand their new partnerships with Latin American stakeholders. Though China’s public and private sectors could provide some funding, investments from Latin American governments and multilateral organizations are also needed. Additional policy support and guidance from the Chinese government, governments in Latin America and international organizations will also encourage and facilitate such collaborations.
Latin America: Capacities, Needs and Concerns
In the context of the external dependency for COVID-19 vaccines faced by Latin-America, China has been one of the most relevant partners to enable countries in the region to access them. Doses procured from and donated by Chinese manufacturers represent more than half of the vaccines administered in the region and play a relevant role, notably in the region’s first efforts to protect health personnel. In Argentina, Brazil and Mexico – some of the few countries in the region with vaccine manufacturing capacity – agreements with Chinese manufacturers also enabled the expansion of vaccination coverage.
Who are the Latin American Stakeholders?
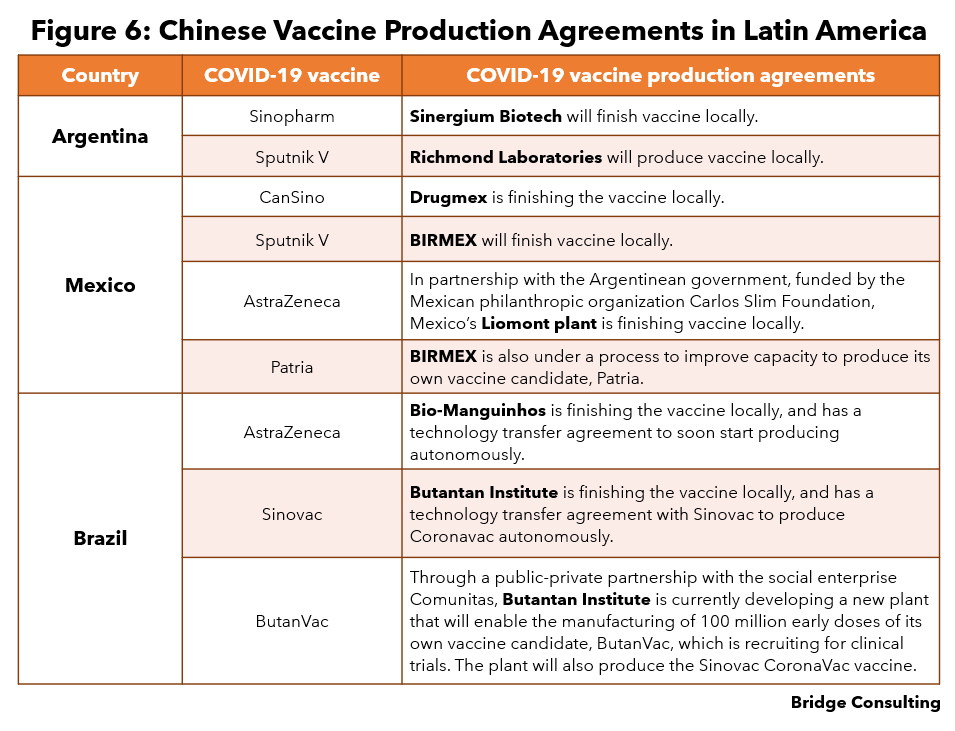
Argentina. Argentina has three main public-led vaccine producers, the Malbán Institute, the Institute of Human Viral Diseases Julio Maiztegui and Sinergium Biotech (Figure 6).
Brazil. Brazil is 54% self-sufficient for its vaccine needs, with production led by three main manufacturers, Bio-Manguinhos, the Butantan Institute and the Technological Institute of Parana. The Butantan Institute has supplied over 50 million Coronavac doses to national immunization efforts and has also expressed its interest in acting as a supplier of COVID–19 vaccines in the region in the future, while also adequately addressing the national demand.
Mexico. Mexico has the second largest pharmaceutical industry in the region and is 25% vaccine self-sufficient. The main national manufacturer is General Biologics and Reactives from Mexico (BIRMEX), a mainly public-led company with the capacity to produce over 100 million vaccine doses per year and responsible for the distribution of vaccines from other producers to meet the national demand. BIRMEX has recently formalized an agreement with Mexico’s National Council of Science and Technology (CONACYT) to improve the company’s capacity.
What are their Concerns and Needs?
The main challenge Latin American countries currently face regarding COVID-19 vaccination is a lack of access to doses that, if maintained, can cause the region to take years to control transmission. Most of the countries in the region lack personnel, research and development capacity to develop vaccines, which is often associated with poorer immunization rates for other vaccines. Following the example of previously mentioned countries that, among few others in Latin America, have manufacturing capacity for vaccines and vaccine components, would take significant public investment in science, technology and infrastructure.
International Stakeholders
To date, almost all COVID-19 vaccine deals and partnerships between China and Latin American countries have been bilateral. While the involvement of international stakeholders has been limited so far, they have expertise in vaccine R&D and production, policy support, and financing, and have potential to play a much bigger role (Figure 7).
What is their Capacity Like?
International standardization. Adhering to international standards for Chinese vaccines will enable their worldwide distribution. International organizations with expertise in vaccine R&D and production such as the CEPI and PATH have supported the R&D of a diverse portfolio of vaccine candidates, and have the potential to facilitate vaccine collaboration between China and Latin America.
Market-shaping. Healthy markets will allow vaccine developers and donors to maximize production and investments based on known demand, making COVID-19 and other vaccines more accessible and affordable for lower-income countries. Gavi, UNICEF and CHAI are three organizations that have had experience working with governments, the private sector, and vaccine manufacturers to negotiate agreements and shape healthy markets. Such organizations can help the stakeholders ensure equitable distribution of COVID-19 and other vaccines in Latin American countries and beyond.
Financing and policy support. Organizations that have experience in financing such as the World Bank, IDB, IMF, and PAHO can help local Latin American stakeholders relieve costs in vaccine procurement and deployment. In particular, the PAHO Revolving Fund for Access to Vaccines has had over 40 years of experience in working with member states to manage their national vaccine supplies.
What are their Concerns and Needs?
Helping Chinese vaccines go global. International organizations that are working towards the equitable distribution of vaccines are constantly concerned about the quality and the reach of vaccines. In order to enable the global distribution, overseas R&D and production of Chinese vaccines, especially in Latin American countries, these international organizations need vaccine developers’ collaboration in sharing and improving their technologies and know-how.
Securing sufficient funding and policy support from involved countries. While some international organizations might specialize in providing funding and policy support, other organizations that specialize in vaccine R&D might not. In order to help with the improvement and transfer of vaccine technologies to other countries, thereby diversifying the supply of vaccine R&D facilities worldwide, these organizations need such support from Chinese, Latin American and other international stakeholders.
The Way Forward
Stakeholders from China, Latin America and international organizations should work towards the same objective, that is to develop solutions to vaccine inequity for COVID-19 and beyond. With that in mind, all stakeholders should:
- Support joint vaccine R&D through mechanisms such as the recently launched BRICS Vaccine Research and Development Centre, the COVID-19 mRNA vaccine technology transfer hub and collaborations between vaccines developers from different regions.
- Build regional vaccine manufacturing hubs by helping certain Latin American countries build up their vaccine manufacturing capacities in order to produce vaccines for the rest of the region, leveraging policy and funding support from a variety of sources, including China and Latin American countries, international organizations and private investors.
References
- Figure 2 sources: CoronaVac, BBIBP-CorV, Ad5-nCoV (Convidecia), RBD-Dimer (ZF2001), Sinopharm (Wuhan) Inactivated (Vero Cells), Chinese Academy of Medical Sciences Inactivated (Vero Cells), Walvax mRNA.
- Figure 3 sources: Argentina, Brazil, Mexico.
- Figure 4 sources: Chile, Manaus, Brazil, São Paulo, Brazil.
- Figure 6 sources: Sinopharm, Sputnik V; CanSino, Sputnik V, AstraZeneca, Patria; AstraZeneca, Sinovac, ButanVac.
About The Authors
Zixiang (George) Zhou
Zixiang (George) Zhou is an experienced international relations and development professional who has worked in Washington DC, Nairobi, and Beijing. Find George on LinkedIn.
Marina Figueira
Marina is a Junior Associate at Global Health Strategies’ Brazil office. With a major in International Relations, her main research and professional interests are international cooperation and public health in Latin America. Find her on Linkedin.
Marisa Lim
Marisa Lim is a Singapore-based aspiring trailblazer majoring in Biomedical Engineering with a specialization in Robotics, passionate about global health and social causes. Find Marisa on LinkedIn.