Opportunities abound: China’s evolving vaccine cooperation and impact in Asia

Source: Xinhua
May 27, 2022 | By Cheng Wei Jian, 2022 Spring Intern, Bridge Consulting
At the 75th World Health Assembly in May 2022, one of the key messages in the area of global pandemic preparedness was the need to diversify vaccine manufacturing across emerging economies. Cooperation in the development of vaccines has become all the more necessary as we consider the desire for global health security and sufficient vaccine supply for both pandemics and routine immunizations across all regions. Under this context it has been interesting to see how vaccine cooperation between China and the rest of Asia has evolved.
Since the start of the pandemic, vaccine cooperation between China and the rest of Asia has been undeniably strong. Looking at the bilateral distribution of Chinese vaccines, China has delivered a total of 1.58 billion doses of COVID-19 vaccines to date, among which Asia received the most out of any continent – almost two-thirds of the doses delivered. Apart from the bilateral distribution to individual Asian countries, many regional initiatives such as the ASEAN-China Vaccine Friend Cooperation have also been implemented to strengthen the cooperation in combatting COVID-19.
However, this cooperation in the form of vaccine deliveries came before China’s shift in focus towards other forms of sustainable partnerships such as fill-to-finish projects and technology transfer, apart from simply supplying vaccines. Indeed, from a timeline of Chinese COVID-19 vaccines delivered to other countries, China has significantly reduced the delivery of vaccine doses in past months. Yet, this does not mean all cooperation between China and other regions have come to a halt. Rather, it signifies the start of China’s new direction for vaccine cooperation to be set in motion.
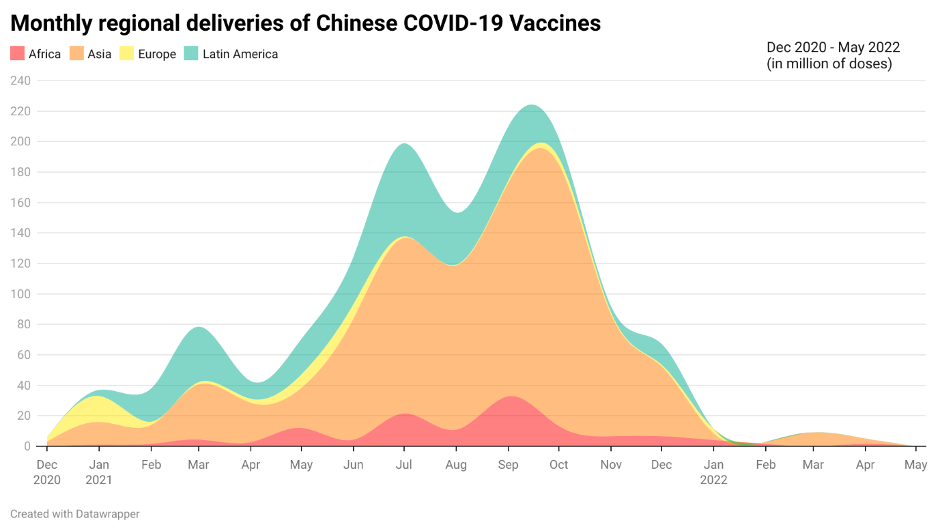
Figure 1: Monthly regional deliveries of Chinese COVID-19 Vaccines
To meet this perceived vaccination demand gap from the reduced vaccine deliveries, mutual support between China and other parts of Asia to improve vaccine research and development (R&D) capacity and capability is more crucial than ever. Surprisingly, contrary to struggling to adapt to this new form of cooperation, cooperation was implemented quickly within the region and created an abundance of vaccine R&D collaboration opportunities for various public or private companies and institutions between China and other Asian countries.
The main reason for this smooth cooperation could be attributed to Asia’s growing capacity for vaccine R&D and production as well as China’s already strong vaccine cooperation within Asia since the onset of the pandemic, especially with their neighboring countries in East and Southeast Asia. This article will focus on how China’s cooperation within these two regions specifically could open doors to developing vaccine R&D around the region and beyond, thus making it necessary to establish the vaccine R&D scene in Asia first before delving into the existing regional cooperation between China and the rest of Asia.
Asia’s growing capacity for vaccine R&D and production
Asia is no novice to handling pandemics. Since the experience of dealing with the Severe Acute Respiratory Syndrome (SARS) back in 2003, investments into building the healthcare and pharmaceutical infrastructures have been growing within Asia.
Since then, Asia has been growing into a potential vaccine hub drawing in multinational pharmaceutical companies that has begun heavily investing into the region. This pouring of investments into the Asia comes as no surprise. Based on an analysis on the pharmaceutical strategy in Asia done by The Economist in 2018, the expected average compound annual growth (CAGR) in pharmaceutical spending by countries in Asia up to 2022 is projected to be 7.63%, up 2.82% from the base years of 2013 to 2017’s average of 4.81%. The significant increase in expected average CAGR in pharmaceutical spending highlights Asia’s willingness to expand their pharmaceutical sector, thus presenting attractive opportunities for foreign companies to enter into the region.
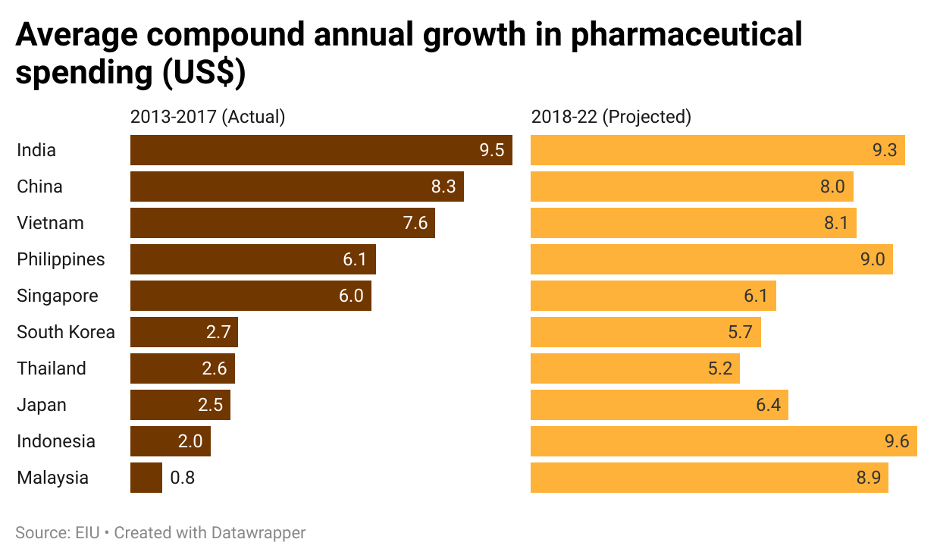
Figure 2: Countries in Asia actual and projected average compound annual growth in pharmaceutical spending (US$)
Regional focus on vaccine R&D
The increase in pharmaceutical spending for East and Southeast Asia however leads to an interesting case on the focus of each region. For instance, within Southeast Asia, much of the foreign investments and development in the pharmaceutical industry lies in building vaccine manufacturing capacity.
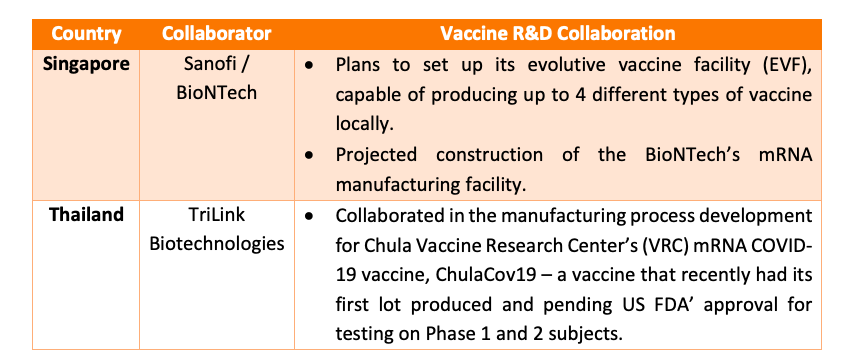
Read more: Sanofi and BioNTech in Singapore; TriLink BioTechnologies in Thailand
This juxtaposes East Asia’s dedication of resources towards the pharmaceutical industry vis-à-vis pandemic preparedness and vaccine R&D.

Read more: Japan’s SCARDA; South Korea’s investment and policy actions initiatives
The growth of vaccine R&D capacity and capability within Asia has also led to a growing involvement in various projects, which can be observed from COVID-19 vaccines R&D projects ongoing within Asia. As of the update on 29 April 2022, the WHO’s COVID-19 vaccine tracker and landscape database for COVID-19 vaccine candidates in development records approximately 35 pre-clinical and 58 clinical vaccine candidates developed by Asian pharmaceutical companies and institutes. This constitutes up to 18% and 38% of the total pre-clinical and clinical vaccines in development respectively, highlighting the vaccine R&D capabilities of Asia, which contributes to a substantial number of vaccines being developed. Much of these findings show that Asia already has the necessary resources and with increasing investments, the vaccine R&D scene in Asia has even more potential to scale up.
Asia’s growing role in global health
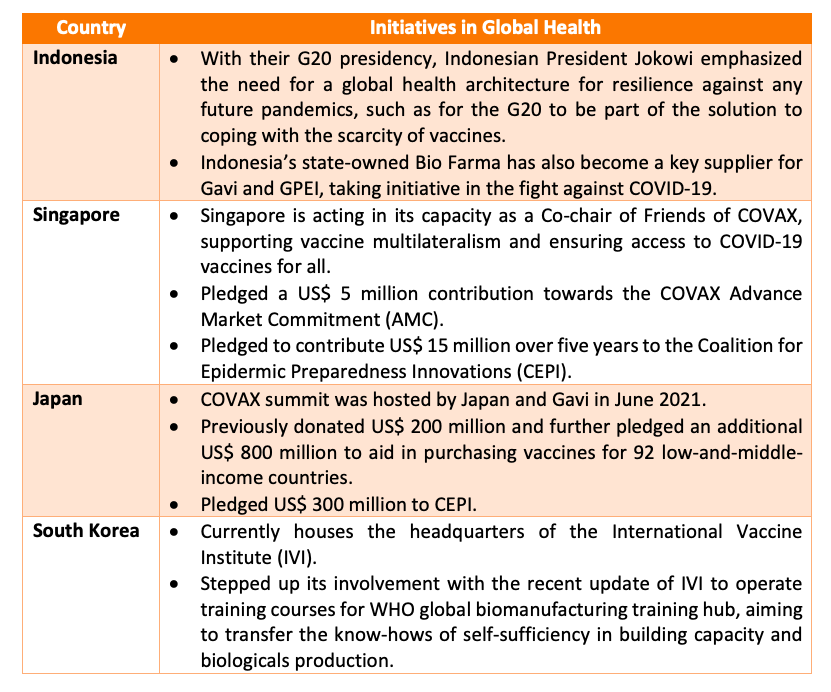
Going beyond the vaccine frontier, some countries within Asia also have large potential to assume more leadership within the global healthcare scene.
Within Southeast Asia, various countries under the ASEAN Vaccine Security and Self-Reliance (AVSSR) have established a Regional Strategic and Action Plan for 2021 to 2025 with the goal of having a shared regional collaboration model and to aim to align with global and regional policies outlined by the Immunization agenda 2030. This pushes ASEAN countries to step up the involvement into global healthcare, by increasing equitable access and use of new and existing vaccines from within the region. East Asia has also been pushing ahead in leading the global healthcare agenda through generous donations supporting the development and production of vaccines for the international community.
From Asia’s vaccine R&D capacity, capability and potential to lead the global healthcare scene, Asia truly has a burgeoning opportunity for growth in further vaccine R&D projects and would be able to benefit with further partnerships to speed up the process. As such, with a better understanding on the context of Asia’s present vaccine R&D, how has a strong vaccine cooperation with China contributed towards meeting the region’s vaccine demands while creating opportunities for Asia’s growth into vaccine R&D?
Regional cooperation in vaccine R&D between China and Asia
Looking ahead to developing a more effective alternative to vaccine cooperation, Chinese President Xi Jinping first proposed the six-point Global Vaccine Cooperation Action Initiative back in October 2021 during an address to the 16th G20 Leaders’ summit. In the first point, President Xi emphasized on strengthening vaccine R&D cooperation and support vaccine companies in conducting joint R&D and production.
With a large part of Asia’s vaccination demand met via China’s supply of vaccines, the shift in focus for vaccine cooperation draws attention to how the cooperation between China and rest of Asia will be able to cope with continued vaccination demand. In response to this, multiple Chinese companies and institutions quickly formed significant partnerships with their Asian counterparts in East and especially Southeast Asia. The swift and effective collaboration helps to sustain a timely and improved accessibility to vaccines around the region and some of these collaborations are illustrated below. Of course, China has also initiated a number of partnerships in South Asia and Central Asia, however, with more limited efforts in vaccine distribution and R&D.
Bilateral cooperation in vaccine distribution
While China has reduced the amount of vaccine distribution from the country, cooperation in vaccine distribution is crucial for helping Asia grow to achieve self-sustainability as a region for countries that may not have the proper infrastructure.
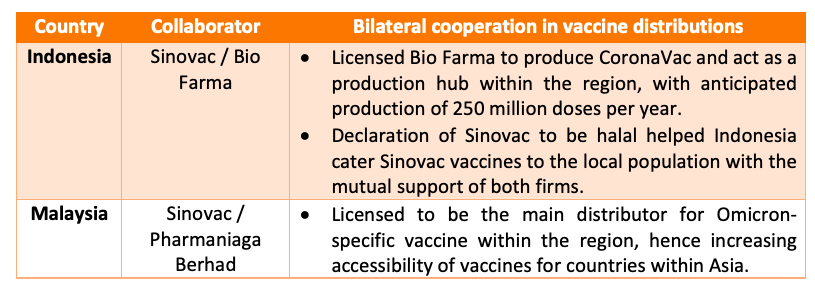
Read more: Sinovac and Bio Farma vaccine distribution partnership and declaration as halal;
Pharmaniaga Berhad as main distributor for Sinovac’s Omicron-specific vaccine
Joint production and vaccine R&D partnerships
Joint productions are also another common type of partnership formed within the region as part of China’s shift to sustainable forms of vaccine cooperation.
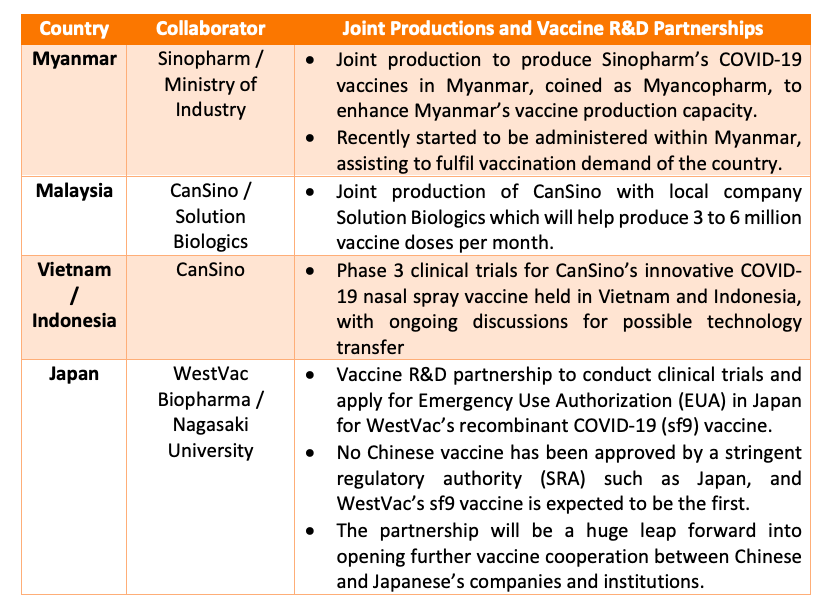
Outside of the aforementioned collaborations, some other private sector investments in R&D for further vaccine R&D in Asian countries are also worth mentioning, such as YishengBio’s acquisition of Singapore’s NewBiomed for the PIKA adjuvant technology. This propelled their R&D capabilities forward to utilize the novel technology and develop multiple vaccines such as the current PIKA recombinant COVID-19 vaccine.
Final thoughts
With the exponential growth of vaccine R&D in these countries, no doubt the region has significant potential to achieve and sustain vaccine self-sufficiency, providing further contributions towards the global healthcare agenda. China with its already strong vaccine cooperation across Asia has also shown the efficiency in which strategic collaborations between the countries can be formed quickly and act as the driving force to accelerate the growth of vaccine R&D capacity and capabilities within the region. Henceforth, the door is now wide open for both sides to strengthen cooperation and lay hold of the opportunities that lie ahead, working towards a robust infrastructure and network for the current and future pandemics.
About The Author
Cheng Wei Jian
Wei Jian is a Singapore-based Research Analyst Intern with an avid interest in the pharmaceutical industry. Having a technical background, he is experienced in handling and visualizing data along with a strong knowledge on global healthcare issues. Find Wei Jian on LinkedIn.



